
FOOD SAFETY MANAGEMENT APPLIED BY DISTRIBUTORS (AGENTS AND BROKERS STANDARDS)
September 24, 2023
WASTE MANAGEMENT FROM THE ASPECT OF FOOD SAFETY STANDARDS
November 13, 2023Unlike other GFSI-recognized standards, the BRC standard raises the level of requirements related to product safety to higher level, by requiring the identification of risk zones, which will ensure that the company’s environmental hygiene standards, especially those related to equipment, buildings, cleaning and personnel hygiene, correspond to the work performed in that specific area.
What are risk zones? “Zones” are defined areas within a production location. “Risk-based zones” (production risk zones) are classified within the BRC standard as follows:
- Open product zones:
• high risk (chilled and frozen)
• high care (chilled and frozen)
• ambient high care
• low risk
- Enclosed product areas (e.g. warehouses and storerooms)
- Non-product areas (e.g. canteens, laundries and offices)
Why is zoning required within the production of food products? According to the BRC standard, the main task of the food product manufacturer is to ensure that the food safety control measures applied in the company are in accordance with the products that are produced in that company. Therefore, where products susceptible to pathogen contamination are handled on site, it is vital to understand the risks and implement effective controls that minimize this potential contamination.
The production location should take into account:
• pathogens to which their products may be susceptible
• the nature of these pathogens, including the likelihood of their survival and/or growth in the product
• the specific controls used to manage these pathogens and how those controls are validated, monitored and consistently maintained.
How to determine risk zones within the company? In version 8 of the BRC standard, there was a decision tree based on which risk zones could be determined:
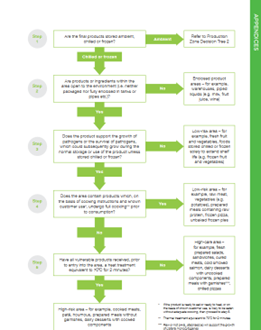
In BRC ver 9 the tree is removed and the zones are determined following the BRC definitions:
Open product areas:
High risk (chilled and frozen)
“This is a physically segregated area (see below) designed to a high-hygiene standard where practices relating to personnel, ingredients, equipment, packaging and the environment aim to prevent contamination by pathogenic micro-organisms. Products which require handling in a high-risk area must meet all of the following criteria:
• The finished products require chilling or freezing during storage to preserve food safety.
• All components have received a full cook7 process to a minimum of 70°C for 2 minutes or equivalent (see Appendix 3) before entry to the area.
• The finished products are vulnerable to the growth of pathogens (e.g. Listeria species) or the survival of pathogens, which could subsequently grow during the normal storage or use of the product (e.g. if a frozen product is defrosted but not immediately consumed).
• The finished products are ready to eat, ready to heat or, on the basis of known consumer use, are likely to be eaten without adequate cooking.
Examples of products considered as high risk include cooked sliced meats and fully cooked prepared meals.”
High care (chilled and frozen)
“This is an area designed to a high standard where practices relating to personnel, ingredients, equipment, packaging and the environment aim to minimise product contamination by pathogenic micro-organisms. Segregation (see below) of the high-care area and access arrangements to the area shall minimise the risk of product contamination.
Products that require handling in a high-care area must meet all of the following criteria:
• The finished products require chilling or freezing during storage (to preserve food safety).
• All microbiologically susceptible components have received a process to reduce the microbiological
contamination to acceptable levels (typically a 1–2 log reduction of micro-organisms such as Listeria species) before entry to the area.
• The finished products are vulnerable to the growth of pathogens or the survival of pathogens, which couldsubsequently grow during the normal storage or use of the product (e.g. if a frozen product is defrosted but not immediately consumed).
• The finished products are ready to eat,8 ready to heat9 or, on the basis of known consumer use, are likely to be eaten without adequate cooking.
Although all vulnerable ingredients and products have, before entry to the high-care area, received a process to reduce pathogenic bacteria to a level to make them safe to eat, spoilage organisms will be present and shall be controlled by temperature and shelf life. Examples of products considered as high care include sandwiches and prepared salads.”
Ambient high care
“This is an area designed to a high standard where practices relating to personnel, ingredients, equipment, packaging and the environment aim to minimise product contamination by pathogenic micro-organisms. Ambient products that are handled in these areas are vulnerable, as pathogens are known to survive on the product. Ambient high-care areas are different from low-risk areas because products handled in low-risk areas either intrinsically, or by design, do not support the growth or survival of pathogens, or are designed to undergo a later validated kill step.
Products which require handling in this area must meet all of the following criteria:
• A raw material is prone to contamination with a vegetative pathogen (e.g. Salmonella species).
• The production process includes a process step which removes or reduces the pathogen (e.g. a microbiological kill step). (Where there is no effective step, it is assumed that any risk associated with the raw material is controlled as part of the raw material risk assessment.)
• The finished products are stored at ambient temperatures (i.e. they are not deliberately temperature-controlled).
• The finished products are ready to eat,ready to heat or, on the basis of known consumer use, are likely to be eaten without adequate cooking.
• The finished products are such that vegetative pathogens could survive and grow in normal use, subsequently causing food poisoning, or are of a nature (e.g. fatty foods) that enables food poisoning to result from a very low level of contamination with a pathogen.
Examples of processes that require an ambient high-care processing area include the manufacture of chocolate from raw cocoa beans, the production of milk powder from raw liquid milk, or the manufacture of peanut butter from raw peanuts”.
Low risk
“The significance to human health of microbiological contamination in low-risk areas is reduced because the products either:
• do not support the growth of pathogens (either intrinsically or by design of the product) or the survival of pathogens, which could subsequently grow during the normal storage or use of the product
• are designed to undergo a later kill step that will ensure the product is safe to eat.
The hygiene standards in such areas generally require greater emphasis on preventing foreign body and allergen contamination, although they will still need to be based on the risks associated with the specific products. Good manufacturing practices, including good process flow, are still expected.Examples of products considered as low risk include raw meat, sugar and flour.”
Detailed explanations related to the determination of zones can be found in the standard BRC ver 9, Appendix 2.
Requirements in BRC Standard ver 9 relating to risk zones:
• 4.3.1. and 4.3.2. – layout, production flow and segregation
• 4.11.7.1. – CIP cleaning
• 8.1.-8.7. – risk zones
If you need help implementing BRC standards, you can contact our agency!!!




