COMPRESSED AIR IN THE FOOD INDUSTRY
January 5, 2023
FOOD FRAUD – FRAUDS RELATED TO FOOD NEW TRENDS
February 13, 2023A product recall is the situation that every company fears the most: in addition to causing large material costs, it also entails the possibility of seriously damaging the brand’s status. However, this does not always have to be the case. There are a large number of cases, especially in recent years, when the recall of a product is a precaution and concern for the potential threat to the health of the consumer, which is certainly recognized as a good manufacturing practice.
Through practice, it can mostly be observed that companies (with reason) find themselves in a dilemma whether a certain situation is a recall or withdrawal, so through this text we will try to clarify this and point out the steps that should be taken in real situations.
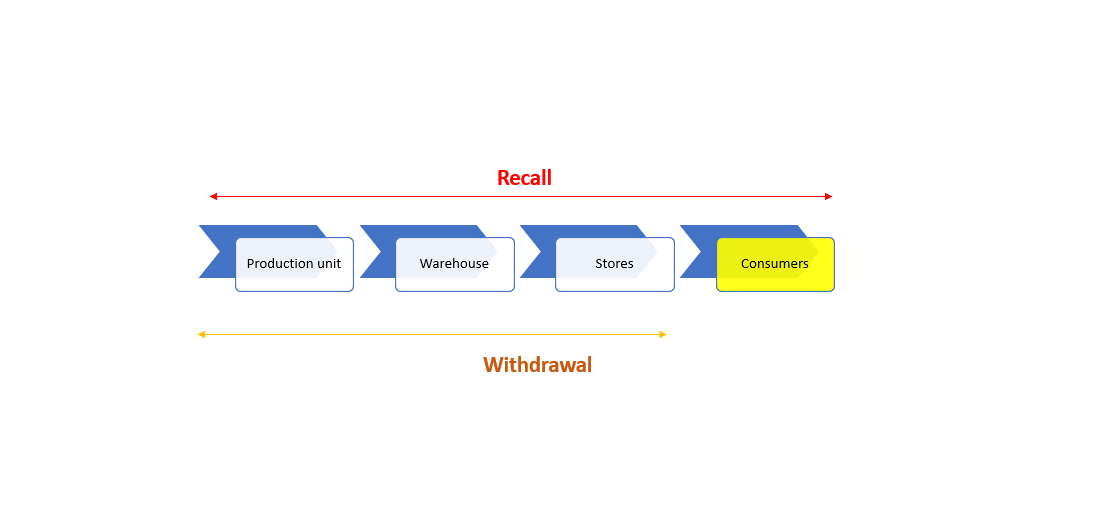
Withdrawal or recall?
Recalls of food products are an important tool that protects consumers from products that are dangerous to health, so manufacturers and distributors are obliged to have effective procedures in place in the event of recalls or recalls. What is a recall and what is a product recall?
Given that the meaning of terms varies between countries and even food safety management system standards and guides, it is no wonder that there is confusion regarding these terms.
According to EU Regulation 178/2002 known as the General Food Law and Directive 2001/95/EC, recall is: “represents any measure that returns an unsafe product that was available to consumers”, while withdrawal is: “any measure that involves preventing distribution , exposure and offer of a dangerous product to consumers”.
The terms “recall” and “withdrawal” have the same meaning according to the legislation of the Republic of Serbia: Law on General Product Safety” (Official Gazette of RS, No. 41/2009 and 77/2019).
On the other hand, according to the FDA (Food and Drug Administration): “a recall is the removal of food from the market that is in violation of the regulations of the US Food and Drug Administration (FDA), while: “withdrawal from the market is a case when the product has a minor violation that would not be subject to FDA legal action. The company removes the product from the market or corrects the violation”.
What do the standards say?
According to the IFS Food standard ver 7,
– recall is “any measure aimed at achieving the return of a dangerous product that has already been delivered or made available to consumers by the manufacturer or distributor”, while
– withdrawal: “Any measure aimed at preventing the distribution, presentation and offer of products that are out of specification and/or products that may be dangerous for the consumer”.
BRC Food standard ver 9 defines:
– product recall as: “All measures aimed at removing inappropriate (eg unsafe) products from buyers and end consumers”.
– product withdrawal as: “All measures aimed at removing from customers, but not final consumers, products that are out of specification or unsuitable (eg unsafe). Recall is usually used to remove products where there is no risk to consumers; for example, when the product has not reached the point of sale to consumers”.
Now all of the above is quite confusing, isn’t it? Which definition to accept as correct and how to distinguish now whether it is a recall or withdrawal of the product? For recall, it is quite clear that it is a procedure for returning products that may harm the health of consumers, while in the case of withdrawal, we rely on the interpretations of the requirements of the applicable legislation and/or the standards applied by the company.
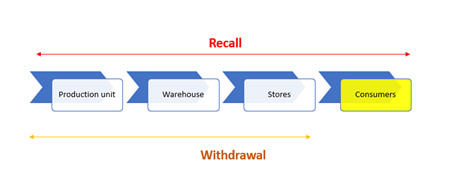
Types of recall. Revocations can be:
- voluntary: when the company itself enters the recall procedure due to perceived problems (or potential problems) or:
- mandatory: when the revocation is ordered by the competent state authorities
Recalls can also be viewed at the level of:
- markets: implies product recall at the level of distribution centers and/or retail stores AND implies food sold in bulk;
- consumer: implies recall from any point of the supply chain: distributors, retailers and consumers;
IMPLEMENTATION OF PRODUCT RECALL AND WITHDRAWAL PROCEDURES
In order to successfully carry out the recall and/or product withdrawal procedure, the company must go through the following stages:
1. Collection and processing of information: This is the key stage in which it is necessary to find out the details of the problem and collect as much information as possible on the basis of which the correct decision will be made whether it is a recall or recall of the product; information is also obtained which product lots should be withdrawn or recalled, identify affected customers and stop further distribution of the product;
2. Notification of: customers, competent state authorities, consumer and certification body (in case of revocation);
3. Monitoring: during the withdrawal or revocation process, be in constant communication with all interested parties;
4. Dealing with unsafe products: unsafe food must be clearly labeled and thereby prevent its further sale and distribution; carry out further handling of the unsafe product according to risk analysis and management system procedures.
Each state’s legislation provides guidelines for notifying state authorities in the event of a product recall. We will compare the revocation procedure in the member states of the European Union and the USA. European Union member states use the crisis management system: RASFF (Rapid Alert System). RASFF enables the rapid exchange of information between the national food safety authorities of each member state, as well as with the European Commission, the European Food Safety Agency and the EFTA (European Free Trade Association), which enables rapid tracking of products to the source; RASFF provides three levels of notifications:
• alerting (does not mean that it necessarily leads to revocation);
• tracking information: causing serious attention from government authorities
• rejection at the EU border.
All notices: including product type, batches, hazard type, etc. are available on the RASFF portal.
Notification system in the USA, involves recall procedure after alerting, public release of photos of recalled product, batch numbers and involved stores/areas. The FDA is involved in the process of disclosing all risks to consumers.
The most common causes of recall of food products
The most common causes of recall of food products are:
-during 2021 In the EU: non-approved ingredients, toxins/chemicals, Salmonella
– during 2021 in the USA: contaminated products, incorrect declarations, undeclared allergens
– in the period 2012-2021. in Australia: undeclared allergens, microbiological contamination, foreign bodies
Although there is always a risk of damage to the brand’s image or material costs in the event of a recall and/or recall, irreversible damage occurs in the event of endangering the health of consumers.