
ALLERGENS – DIFFERENCES IN EU/US LEGAL REQUIREMENTS
July 7, 2023
PREREQUISITE PROGRAMS (PRPs)
July 29, 2023We spoke about the quality of drinking water in the food industry in the text: https://asconsulting.rs/water-in-the-food-industry/. Now we continue to clarify this topic by dealing with drinking water quality parameters.
Water quality indicators can be: physical, chemical and sanitary. According to the “Rulebook on the hygienic suitability of drinking water” which is applied in our country, the quality criteria for drinking water are: physical, physico-chemical, chemical and microbiological.

Physical properties of water
The physical properties of water are: temperature, color, odor, taste, turbidity, residual solids and conductivity.
Water temperature: The most suitable temperature for drinking water is 8-12˚C. As the temperature rises, water loses its drinkability, more is drunk to quench thirst, which can have a harmful effect on the digestive tract. Colder water is unfriendly to the mucous membrane of the pharynx and digestive tract.
Water color: The color of water most often originates from humic substances. It has no major sanitary significance, although it is not desirable for aesthetic reasons. The limit value for the intensity of drinking water should be 10-20˚ of the cobalt-platinum scale, i.e. up to 20 mg/l of KMnO consumption if the water contains humic substances.
The smell and taste of water. Odor and taste are caused by the presence of microorganisms, dead or alive; dissolved gases such as hydrogen sulfide, methane, carbon dioxide or oxygen; organic matter; mineral substances such as sodium chloride, iron compounds, sulfates and carbonates of other elements; phenols and other tar substances, especially after chlorination. Drinking water should be odorless and tasteless, i.e. it must have a pleasant, refreshing taste.
Water turbidity: Turbidity is caused by the content of suspended and colloidal substances. Turbidity is characteristic of surface, karst and shallow groundwater. Cloudy water is bad for drinking and some industrial processes (food and drink production).
Residual solids: These are the substances that remain after evaporation, because there is always a greater or less amount of dissolved or suspended substances in the water. Water with a large amount of residual residue (hard water) is not suitable for industrial use.
Conductivity: An increase in conductivity indicates some pollution; information on the concentration of total dissolved substances (degree of water mineralization) can be obtained through the measured values of the electrical conductivity of water.

Chemical properties of water
Unlike the physical properties, the chemical properties of water are more diverse and more important for the immediate assessment of quality. Any chemical substance can be a poison, which depends on the concentration, length of ingestion and properties of the substance itself. The following are used to assess water quality: active reaction, hardness, oxidizability (redox potential), etc.
Active reaction: An active reaction is characterized by the acidity or alkalinity of water. It is evaluated by the degree of hydrogen-ion concentration (pH). In a neutral reaction, pH = 7, in a basic reaction, pH > 7 and in an acidic reaction, pH < 7. The value of pH for drinking water, it should range from 6.5 to 9.5 (according to our regulations, 6.8 to 8.5).
Water hardness: Water hardness is determined by the presence of dissolved calcium and magnesium salts. Dissolved salts in water change its properties and cause unwanted effects when using water (for example, they create scale on steam boilers or react with the ingredients of basic raw materials to give insoluble products that change the quality of finished products). We distinguish between carbonate hardness (transient, which originates from metal ions bound in the form of bicarbonate) and non-carbonate hardness (permanent) due to a certain amount of non-carbonate salts (chlorides, sulfates and nitrates of calcium and magnesium). Hard water is undesirable in industry due to higher fuel consumption for heating boilers, forming stones, etc. Otherwise, it is considered that they do not pose a danger to human health.
Oxidativeness (redox potential): Oxidativeness is the total content of pollutants (organic and inorganic) that react with strong oxidants. Oxidativeness is determined in (mg/l) oxygen required for pollutant oxidation.
Nitrogen compounds: Nitrogen compounds present in water indicate the presence of organic matter.
Chlorides: Chlorides present in drinking water in concentrations higher than permitted (200 mg/l) are a sign of water pollution (current or permanent water pollution by faeces or industry), with the exception of chlorides of inorganic origin – salt water.
Halides: Chlorine is not found in dissolved form in natural waters. Its presence in purified water originates from the use of chlorine as a disinfectant in the form of residual chlorine. Fluoride is present in many natural drinking waters. Iodides are rarely found in natural waters in quantities that would constitute a sanitary nuisance.
Phosphates: Phosphates are not significantly present in natural waters. However, the use of polyphosphates for corrosion protection can lead to phosphates being found in drinking water.
Asbestos: Asbestos must not be in drinking water (natural) that is bottled because it causes cancer.
Phenol: Phenol present in water indicates its pollution by industrial waste.
Mineral oils: Mineral oils are a mixture of various organic compounds of unknown composition.
Toxic substances: Metal ions in water are toxic and represent poisonous substances. These are: lead, zinc, copper, chromium and mercury. Some of the toxic components act immediately, while others accumulate in certain organs (tissues) and act later, when they exceed a critical amount. Such substances include: pesticides, war poisons, radioactive substances, polycyclic aromatic hydrocarbons, manganese, etc.

Microbiological properties of water
The microbiological properties of water can be said to represent the most important indicator of water quality due to the effects on human health, physical and chemical quality of water and finally the choice of water purification procedures. Microorganisms living in water can be responsible for the occurrence of health problems in consumers of the given water, including infections caused by bacteria. Because of all this, the greatest attention is paid to the detection and removal of these organisms from water.
Primary interest groups include bacteria, viruses, algae, protozoa and intestinal worms.
Bacteria are unicellular organisms between 0.1 and 10 μm in size, invisible to the eye. In raw water, sunlight, thermal effects, sedimentation and predators have an effect on reducing the number of bacteria. Purified rivers represent the best medium, most likely due to the presence of organic matter and the lack of predators. It is true that survival of these organisms is facilitated at lower temperatures. The content of bacteria in drinking water is the most important hygienic indicator of water quality. Water is polluted with pathogens and other microorganisms through fecal pollution of human and animal origin, then through waste from rural settlements. Due to the direct danger to the health of the population, drinking water must not contain such microorganisms.
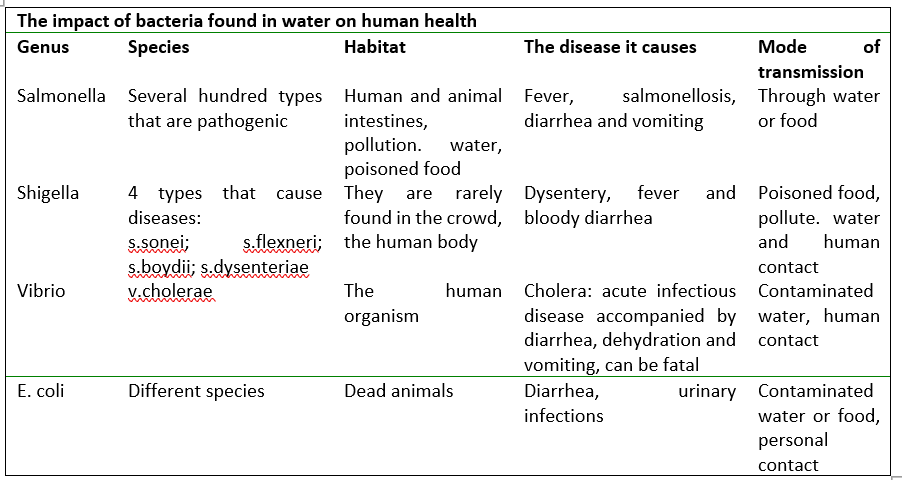
The problem of bacteria in water is solved by procedures used in the preparation of drinking water: the largest percentage of bacteria is removed during coagulation and filtration, and the final treatment involves disinfection.
Indicators of total faecal water pollution (aerobic mesophilic bacteria, total coliform and sulfite-reducing clostridia are allowed depending on the type and importance of water (purified water, disinfected, bottled and natural – raw water).
Since pathogenic bacteria are difficult to prove in drinking water, only indicators of fecal pollution are investigated, and only when a deviation from microbiological characteristics is established on the basis of a basic or extended examination, then pathogenic organisms of Salmonella and Shigella species are sought.
Viruses are simple organisms in structure; they are the smallest microorganisms, ranging in size from several tens to several hundred millimicrons. All are parasites and can be found in animals, plants, bacteria, fungi and algae. Viruses can be found in water through direct transmission by humans or animals or indirectly from rural or urban areas. Important factors in water that make it difficult for viruses to develop are temperature, sunlight and drought. Important chemical factors include pH, heavy metals, and oxidizing agents such as chlorine, ozone, bromine, and iodine. Factors that accelerate the development of viruses in water are suspended solids and turbidity. They are removed from the water by slow sand filters.
Algae are a group of lower plants of various forms; they mostly live in water and play an important role in reservoirs and lakes in the process of circulating nutrients and other factors as part of the food chain, they serve as food for zooplankton and fish. Many are single-celled; they consist of one spherical or differently shaped cells. Several species are pathogenic to humans, producing endotoxins that cause gastroerinitis. Algae that pass the preliminary water treatment and are retained on the filters will quickly die there. In addition, effective coagulation and sedimentation removes 90-95 percent of algae. In slow sand filters, algae can enhance removal by forming a surface layer on the filter where smaller impurities are retained.
Protozoa are a group of unicellular organisms that do not have the ability to carry out photosynthesis. Most are mobile, several species are parasites and five species are human parasites that enter the body through polluted water. Method of removing protozoa from water: more than 99% can be removed by filtration, the rest is removed by coagulation and chlorination – the necessary amount of residual chlorine for complete removal is 0.2 mg/l at 22-25˚C and pH = 6.




